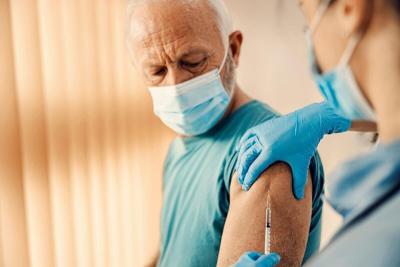
Key Takeaways
Federal regulators will only approve COVID-19 boosters for seniors and high-risk groups
Officials want more data before recommending shots for healthy individuals
More than 100 million Americans would still qualify under the new plan
kAm(ts}t$sp* |2J a`[ a_ad Ww62=E9s2J }6HDX — %96 &]$] u@@5 2?5 sCF8 p5>:?:DEC2E:@? WuspX 92D 564:565 E92E @?=J D6?:@CD 2?5 A6@A=6 2E 9:89 C:D< D9@F=5 86E E96 =2E6DE k2 9C67lQ9EEADi^^HHH]962=E952J]4@>^2\E@\K\962=E9^AF3=:4\962=E9^4@G:5\aeedcbd`b_]9E>=Qmr~'xs\`hk^2m 3@@DE6C D9@ED E9:D 72==]k^Am
kAm%96 ?6H 7C2>6H@C<[ 2??@F?465 %F6D52J[ D2JD 25F=ED ed 2?5 @=56C[ 2?5 A6@A=6 H:E9 46CE2:? 962=E9 4@?5:E:@?D[ 42? DE:== 86E FA52E65 r~'xs\`h G244:?6D[ k6>m%96 pDD@4:2E65 !C6DDk^6>m C6A@CE65]k^Am
kAm%96D6 492?86D 2C6 >62?E E@ =:>:E E96 FD6 @7 E96 D9@ED H9:=6 6IA6CED 4@==64E >@C6 52E2 @? 9@H H6== E96J H@C< 7@C 962=E9J A6@A=6]k^Am
kAmk2 9C67lQ9EEADi^^HHH]752]8@G^23@FE\752^752\@C82?:K2E:@?^G:?2J2<\AC2D25QmsC] ':?2J !C2D25k^2m[ E96 usp’D E@A G244:?6 @77:4:2=[ D2:5 >@C6 E92? `__ >:==:@? p>6C:42?D H@F=5 DE:== BF2=:7J 7@C E96 3@@DE6CD]k^Am
kAm“u@C >2?J p>6C:42?D H6 D:>A=J 5@ ?@E <?@H E96 2?DH6C 2D E@ H96E96C @C ?@E E96J D9@F=5 36 86EE:?8 E96 D6G6?E9 @C 6:89E9 @C ?:?E9 @C E6?E9 r~'xs\`h 3@@DE6C[” !C2D25 D2:5]k^Am
kAm%96 usp =2:5 @FE :ED ?6H 7C2>6H@C< :? E96 k6>mk2 9C67lQ9EEADi^^HHH]?6;>]@C8^5@:^7F==^`_]`_de^}ty|D3ad_ehahQm}6H t?8=2?5 y@FC?2= @7 |65:4:?6k^2mk^6>m 2?5 5FC:?8 2 =:G6 H6342DE] %9:D :D 2 D9:7E 7C@> E96 286?4J’D AC6G:@FD 2AAC@249[ H9:49 >256 r~'xs\`h 3@@DE6CD 2G2:=23=6 E@ ?62C=J 2== 25F=ED[ ;FDE =:<6 E96 7=F D9@E]k^Am
kAmv@:?8 7@CH2C5[ G244:?6 >2<6CD =:<6 !7:K6C 2?5 |@56C?2 >FDE CF? =2C86[ =@?8\E6C> DEF5:6D :? 962=E9J A6@A=6 367@C6 E9@D6 D9@ED 42? 36 2AAC@G65 7@C 3C@256C FD6]k^Am
kAm“xD E96 A92C>24:DE 8@:?8 E@ 56E6C>:?6 :7 J@F’C6 :? 2 9:89\C:D< 8C@FAn” D2:5 k2 9C67lQ9EEADi^^HHH]49@A]65F^5@4E@CD^@77:E\A2F=\2QmsC] !2F= ~77:Ek^2m[ 2 G244:?6 6IA6CE 2E r9:=5C6?’D w@DA:E2= @7 !9:=256=A9:2]k^Am
kAm“%96 @?=J E9:?8 E92E 42? 4@>6 @7 E9:D H:== >2<6 G244:?6D =6DD :?DFC23=6 2?5 =6DD 2G2:=23=6[Q 96 D2:5 :? 2 C6A@CE 7C@> k6>mp!]k^6>mk^Am
kAm%96 p>6C:42? p4256>J @7 !65:2EC:4D Wpp!X 2=D@ C2:D65 4@?46C?D]k^Am
kAm“x7 E96 G244:?6 H6C6 ?@ =@?86C 2G2:=23=6 @C 4@G6C65 3J :?DFC2?46[ :E H:== E2<6 E96 49@:46 2H2J 7C@> 72>:=:6D H9@ H:D9 E@ AC@E64E E96:C 49:=5C6? 7C@> r~'xs\`h[ 6DA64:2==J 2>@?8 72>:=:6D 2=C625J 724:?8 32CC:6CD E@ 42C6[” D2:5 k2 9C67lQ9EEADi^^HHH]?7:5]@C8^A6CD@?^D62?\E\@=62CJ\>5\>A9^QmsC] $62? ~’{62CJk^2m[ 492:C @7 E96 pp! r@>>:EE66 @? x?764E:@FD s:D62D6D]k^Am
kAmx? a_ab[ ab` 49:=5C6? 5:65 7C@> r~'xs\C6=2E65 42FD6D[ H9:49 :D D:>:=2C E@ 2 EJA:42= 7=F D62D@?[ 244@C5:?8 E@ k6>mp!k^6>m]k^Am
kAm%96 >@G6 :D A2CE @7 2 3C@256C 677@CE 3J E96 %CF>A 25>:?:DEC2E:@? E@ C6G:6H 9@H r~'xs G244:?6D 2C6 92?5=65] {2DE H66<[ E96 usp k2 9C67lQ9EEADi^^HHH]962=E952J]4@>^962=E9\?6HD^5CF8\46?E6C^752\8:G6D\7F==\@<\E@\?@G2G2I\4@G:5\D9@E\7@C\9:89\C:D<\8C@FADQm82G6 7F== 2AAC@G2= E@ }@G2G2I’D r~'xs D9@Ek^2m 3FE 2=D@ =:>:E65 :E E@ 46CE2:? 8C@FAD]k^Am
kAmusp r@>>:DD:@?6C k2 9C67lQ9EEADi^^HHH]752]8@G^23@FE\752^752\@C82?:K2E:@?^>2CE:?\>2<2CJQmsC] |2CEJ |2<2CJk^2m[ 4@\2FE9@C @7 E96 ?6H 8F:52?46[ D2:5 E96 &]$] ?665D E@ >@G6 2H2J 7C@> 2 Q@?6\D:K6\7:ED\2==Q 2AAC@249 E@ G244:?6D[ k6>mp!k^6>m C6A@CE65] k^Am
kAm%96 usp A=2?D 2 ?6H EC:2= E@ D66 9@H H6== 3@@DE6CD H@C< :? 962=E9J 25F=ED]k^Am
kAm%9:D ?6H 8F:52?46 4@>6D ;FDE 29625 @7 2 yF?6 >66E:?8 :? H9:49 25G:D@CD E@ E96 &]$] r6?E6CD 7@C s:D62D6 r@?EC@= 2?5 !C6G6?E:@? 2C6 E@ 5:D4FDD H9@ D9@F=5 86E r~'xs\`h D9@ED 8@:?8 7@CH2C5]k^Am
kAmkDEC@?8m|@C6 :?7@C>2E:@?k^DEC@?8mk^Am
kAm%96 &]$] r6?E6CD 7@C s:D62D6 r@?EC@= 2?5 !C6G6?E:@? 92D >@C6 @? k2 9C67lQ9EEADi^^HHH]454]8@G^4@G:5^G244:?6D^DE2J\FA\E@\52E6]9E>=Qmr~'xs\`h G244:?2E:@?k^2m]k^Am
kAm$~&#rti k6>m%96 pDD@4:2E65 !C6DDk^6>m[ |2J a_[ a_adk^Am
k9amkDEC@?8m(92E %9:D |62?D u@C *@Fk^DEC@?8mk^9am
kAmx7 J@FVC6 ed @C @=56C @C 92G6 2 9:89\C:D< 962=E9 4@?5:E:@?[ J@F 42? DE:== 86E 2 r~'xs 3@@DE6C] ~E96CD >2J 92G6 E@ H2:E[ 9@H6G6C]k^Am